ISPE’s Pharma 4.0 initiative points to everything the pharmaceutical industry could be if it embraced the same advancements of the fourth industrial revolution: digitization, empowerment, and automation. This is achieved through the integration of Industry 4.0 technology, originally spurred by the internet and now encompassing artificial intelligence, advanced robotics, and automation. Pharma 4.0 is sometimes referred to as Smart Factory.
So where exactly do environmental monitoring systems (EMS) come into play? For many pharmaceutical organizations, cleanrooms are at the heart of production and research and the EMS is what makes the cleanroom what it is: clean. The impact a well-designed and integrated EMS can have on an organization directly correlates to the organization’s ability to transition to Pharma 4.0.
Regulatory Compliance
Pharma 4.0 accounts for regulations and compliance, as they are ever increasing in our complex world. With a solid EMS, regulatory compliance is literally built into the design of the system. They track events, SOPs, and responsible persons and make certification and audits a breeze. They have the ability to be adapted and customized for your unique needs.
Agility and Adaptation
The fast-moving organizations best suited for Pharma 4.0 need to be agile and adaptable, as the rapidly evolving technology can quickly change the landscape of the industry. A customizable and high quality EMS will be able to adapt to the needs of the organization as they change.
Data Integrity
One of the cornerstones of a good EMS is excellent data integrity – and it also plays a critical role in Pharma 4.0. Organizations transitioning into Pharma 4.0 consider data integrity from the first phase of design.
The FDA defines data integrity as the “completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA).” In order for an organization to make data-driven and well-informed decisions, they have to trust their data. Data is only trustworthy when top-notch data integrity protocols are in place.
Moreover, data integrity plays an important role in end-user safety and regulatory compliance and audits. These are all foundations of organizations operating in Pharma 4.0.
Traceability
A part of data integrity, traceability plays an important role in Pharma 4.0, as well as an EMS. Traceability equates to responsibility and accountability. In cleanrooms operating on paper, traceability is often questionable. In rushed moments, mistakes can be made or information flat-out missed.
An EMS designed for Pharma 4.0 improves on and accounts for traceability.
Real-Time Data Driven Decisions
If there is one thing that is at the heart of Pharma 4.0, it’s innovation. But if there is one tool that makes innovation possible, it’s data that can drive decisions. Information access is incredibly important in Pharma 4.0. In fact, one of the foundations of Pharma 4.0 is increasing access to information and empowering employees to make more informed decisions without running them through management. This enables agility and adaptation.
As far as an EMS is concerned, this means making sure information is not only accurate but readily available for decision making. This equates to continuous monitoring and reporting. For cleanrooms who only take occasional contamination readings, it means you only have occasional information which means you can only make informed decisions occasionally. Continuous monitoring is the future – and already the standard.
Holistic Control Strategy
ISPE defines a holistic control strategy as one that “incorporates GMP and Good Distribution Practices (GDP) and defines the controls from a holistic perspective that will ensure product quality and clinical performance, product availability, and product improvement.” Where a cleanroom is concerned, ensuring product quality does involve monitoring the environment. Without ensuring zero contamination, the product cannot be considered sound. Thus, an EMS plays an important role in this strategy.
An EMS designed for Pharma 4.0 takes this holistic approach into account and incorporates GMP and GDP from the start.
How Can You Transition Into Pharma 4.0?
To transition into Pharma 4.0, you first need to understand the power that comes from an organization which operates on empowerment, agility, information access, automation, and advanced technology. When you and your team can embrace this future, you will need to start investing in the technology that can make this future into a reality.
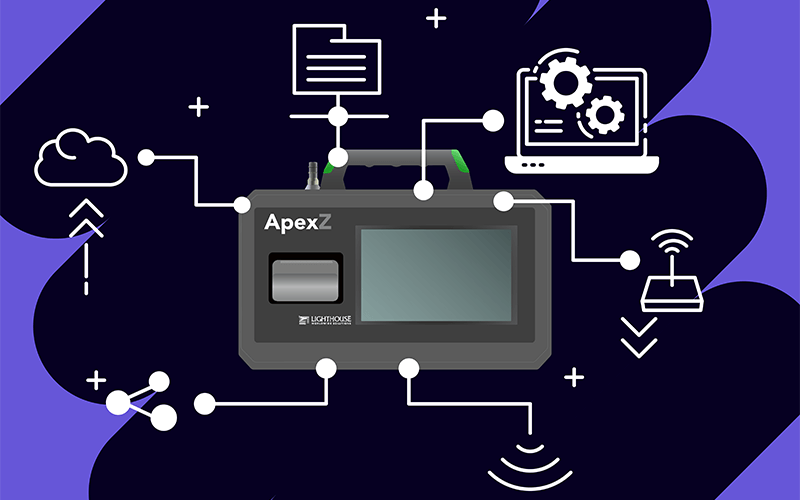
For an EMS, this starts with a solid network of particle counters. In many cleanrooms, this involves a strategic combination of remote particle counters and portable particle counters. Remote particle counters are more permanent than their portable counterparts, designed to stay in the same place and offer continuous monitoring. They do not have displays on the particle counter and, instead, only offer readouts to computers.
On the other hand, portable particle counters have a display on the particle counter and can be moved around the cleanroom. They are especially useful when it comes to classification as they can be moved to different areas of the cleanroom for readings.
In complex cleanrooms, both serve their purpose and can be used accordingly. For smaller cleanrooms, a single portable particle counter might be enough. But one thing is for certain: the particle counters, serving as the foundation of the EMS, need to be designed for Pharma 4.0.
For example, take LWS’ ApexZ, the portable particle counter that sets industry standards for battery life, ergonomics, and advanced self-diagnostics. It is LWS’ take on what a Pharma 4.0 EMS should look like: self-sufficient, effective, adaptable, smart, consistent, easily integrated, and powerful.