


Ensuring Cleanliness and Product Quality Keeping precision parts free from contamination is crucial in industries like aerospace, automotive, semiconductors, and medical devices. Even the smallest particles can impact performance, reduce product lifespan, or, in the case of medical implants, pose serious health risks. To maintain high cleanliness standards, manufacturers use strict cleaning processes, often shifting […]

In today’s world, precision and cleanliness are critical in industries like pharmaceuticals and biotechnology. Among the many tools used to maintain strict cleanliness standards, viable air samplers stand out as a key player. But why are these devices so essential? Let’s dive into the reasons. What are Viable Particle Counters? Viable air samplers are specialized […]
Liquid particle counters are like silent guardians in industries where fluid purity is critical. From pharmaceuticals to water treatment and semiconductor manufacturing, these devices ensure that tiny, often invisible particles don’t compromise product quality, safety, or efficiency. Let’s break down how they work, where they’re used, and what makes them so important. How Liquid Particle […]
Cleanrooms are essential controlled environments used in industries such as pharmaceuticals, biotechnology, and electronics manufacturing. Their primary purpose is to maintain high levels of cleanliness and minimize contamination, which is critical for ensuring product quality and compliance with stringent regulations. Recent advancements are providing fresh insights into how we can enhance cleanroom efficiency, and companies […]
Particle monitoring is like having a super-sensitive detective on your team, keeping an eye on invisible contaminants that could disrupt quality, safety, or efficiency. As industries evolve and regulations tighten, the need for precise monitoring systems has never been greater. Let’s explore why and how different industries rely on this essential technology. Why Is Particle […]
At Lighthouse Worldwide Solutions, we’re excited to introduce our latest innovation: the Active Count Remote Impactor Kit. This advanced system pairs the trusted performance of our ActiveCount25H or ActiveCount100H viable samplers with the newly developed Remote Impactor Kit. Designed specifically for cleanrooms and aseptic environments, this solution offers unmatched portability and flexibility, making sampling easier, […]

In 2024 our knowledge center webinar series provided a comprehensive exploration into the latest advancements and best practices in cleanroom monitoring and environmental control. Covering a wide array of topics, from fundamental techniques to cutting-edge technologies, these sessions aimed to equip professionals with the knowledge and tools necessary to maintain compliance and ensure optimal conditions […]
Ultra Pure Water (UPW) is critical in industries where even the smallest impurities can have significant consequences. It is used in semiconductor manufacturing, pharmaceuticals, and high-end laboratories—applications that demand the highest level of purity. Achieving and maintaining this purity requires a complex process and advanced monitoring tools. This blog explores the UPW process and highlights […]
At Lighthouse Worldwide Solutions, we’re excited to introduce our latest innovation: the ActiveCount Remote Impactor Kit. This advanced system pairs the trusted performance of our ActiveCount25H or ActiveCount100H viable samplers with the newly developed Remote Impactor Kit. Designed specifically for cleanrooms and aseptic environments, this solution offers unmatched portability and flexibility, making sampling easier, even […]
Liquid particle counting is essential for maintaining public health and safety by monitoring the purity and quality of liquids across various sectors, including drinking water, pharmaceuticals, and food production. This technology helps detect contaminants that could pose health risks, ensuring that products meet strict safety standards and protect consumers. Protecting Drinking Water Quality A key […]



It’s actually not as complicated as it might sound at first. Think of GAMP as your friendly neighborhood guide to making sure medicines are top-notch and safe for everyone. GAMP stands for Good Automated Manufacturing Practice. It’s like a rulebook created by a group of pharmaceutical experts who want to make sure that when we use […]



Airborne Particle Counters (APCs) play a critical role in monitoring and maintaining the cleanliness of environments where air purity is essential, such as cleanrooms. There are two fundamental types of APCs: portable and fixed-point remote. Each type has unique features and applications, making them suitable for various scenarios in contamination control. Portable APCs are self-contained […]
In a cleanroom, even the smallest particle can pose significant risks. Particle deposition occurs when airborne particles come to rest on surfaces. Particle deposition in cleanrooms can compromise sensitive processes, contaminate products, and affect the precision of experiments. Have you ever wondered how tiny particles floating in the air end up settling on surfaces like […]
Ensuring that the equipment used within cleanrooms is easy to wipe down and free from particle contamination is vital. Poorly designed equipment can become a contamination source, which is ironic given that such equipment is intended to monitor contamination levels. At Lighthouse Worldwide Solutions, we designed the ApexZ particle counter to facilitate easy cleaning, thereby […]

Gas Sampling in Cleanrooms In cleanrooms, especially those classified ISO 5 or cleaner, maintaining contamination control is paramount. One significant potential source of contamination is compressed gas. Proper gas sampling is crucial in these environments to ensure that the compressed gases used do not introduce particles that could compromise the cleanroom’s integrity. The international standard […]
Overview Cleanrooms are essential in the pharmaceutical industry, providing controlled environments for the aseptic production of sterile products. Recently, big data analytics has emerged as a transformative tool, offering new ways to enhance cleanroom performance beyond traditional methods. The Advent of Big Data Analytics in Cleanrooms Big data analytics involves examining large and varied data […]




Understanding High Pressure Diffusers High Pressure Diffusers (HPDs) are essential for maintaining the precision of particle counts in compressed gas samples. They act as a link between the particle counter and the compressed gas line, diffusing the gas as it enters the particle counter’s sample inlet. This ensures that the pressure is controlled to prevent […]
Understanding High Pressure Diffusers High Pressure Diffusers (HPDs) play a crucial role in ensuring the accuracy of your particle counts when dealing with compressed gas samples. These devices serve as the bridge between the particle counter and the compressed gas line – diffusing the gas as it enters the particle counter sample inlet so the […]
As our world steadily moves towards a future dominated by electric vehicles (EVs), the safe and efficient manufacturing of EV batteries becomes increasingly critical. However, lurking within this crucial process lies a significant challenge: particle contamination. So, let’s explore the implications of this hidden challenge in EV battery manufacturing and how it affects everything from […]


In the realm of cleanroom operations, humans are both a crucial asset and a potential source of contamination. Understanding and mitigating human factors are essential for maintaining optimal cleanliness and efficiency in sterile manufacturing processes. 1. Human-Induced Contamination: Humans are significant producers of particles within cleanroom environments, shedding up to 40,000 skin cells per minute and […]
In the meticulous world of cleanroom operations, efficiency isn’t just a goal—it’s a necessity. From real-time monitoring to paperless processes, here are three key strategies to elevate workflow and streamline operations. 1. Real-time Monitoring: Contamination is inevitable, but swift detection and response can mitigate its impact. Real-time monitoring and continuous microbial monitoring bridge the gap between data collection and analysis, […]
In the realm of sterile manufacturing processes, the Environmental Monitoring System (EMS) serves as a crucial barrier between success and potential disasters like system failures, product recalls, and contamination, which could lead to product shortages. Selecting the right environmental monitoring software and hardware is paramount to the success of your cleanroom operations. The software serves as the […]

In the world of pharmaceutical manufacturing, biotechnology, semiconductor fabrication, and cleanroom environments, precision monitoring is critical to ensuring the effectiveness and safety of quality products. The accuracy of your monitoring data is paramount to avoiding errors and losses during production. False count rate, also known as zero-count rate, is a crucial metric used to assess the […]
In the rapidly evolving landscape of pharmaceutical manufacturing, the integration of cutting-edge technologies is not just a trend but a necessity to meet the stringent regulatory standards and ensure the highest quality of products. The advent of Pharma 4.0 has ushered in a new era of digital transformation, leveraging the power of the Internet of […]
Introducing a groundbreaking revolution in the world of liquid particle counting, we are thrilled to announce the latest addition to the Vertex family of LWS liquid particle counters – the Vertex100. The Vertex100 redefines the way you manage particle counting with its incredibly compact design, unparalleled sensitivity, and true real-time data. The Original Innovation The […]


In the world of cleanrooms, where the highest standards of cleanliness and contamination control are paramount, air filtration systems play a pivotal role. The evolution of these systems, especially with the integration of advanced features, has significantly improved compliance with stringent industry standards. In this blog, we delve into the intricacies of these advanced air […]


The rapid rise of electric vehicles (EVs) has brought about a surge in demand for high-quality lithium-ion batteries. These batteries are the heart of EVs, providing the power needed to propel these vehicles. However, manufacturing lithium-ion batteries is a complex process that demands meticulous control over various factors, including particle contamination. Particle Contamination: A Hidden […]



The 2022 update of the EU GMP Annex 1 has placed significant emphasis on the concept of continuous environmental monitoring (EM), highlighting it as a critical component in reducing the risk of contamination during the manufacture of sterile products. This shift towards continuous EM requires manufacturers to adopt specific techniques and align their monitoring programs […]
Air Sampler impaction technology has been around for decades. However, the d50 is a little known aspect of air sampler technology design and it plays a critical role in air sample capture. Aerosol Impaction is the process in which particles are removed from an air stream by forcing the gases to make a sharp bend. […]
Introduction In the realm of pharmaceuticals, the pursuit of excellence in product quality is a never-ending journey. Governments and private industries have diligently crafted recommendations and regulations over the years, evolving in response to the dynamic landscape of the pharmaceutical sector, particularly in the digital age. In this comprehensive paper, we delve into the fundamental […]
Introduction Cleanrooms in the Pharmaceutical industry are sanctuaries of precision, where the highest standards of cleanliness are not just expected but demanded. A key player in the sterilization arsenal of these cleanrooms is Vaporized Hydrogen Peroxide (VHP), a potent chemical used to disinfect surfaces and equipment. However, the nature of VHP, while highly effective at […]
Introduction In the high-stakes realm of Pharmaceutical manufacturing, precision and adherence to stringent cleanliness standards are non-negotiable. Compressed gases are a staple in cleanroom environments, but they also present a potential risk of contamination. This article delves into the pivotal role of compressed gas sampling in pharma cleanrooms, emphasizing the unique challenges and regulatory considerations […]
Introduction Airborne particle counters play a crucial role in ensuring the environmental integrity of pharmaceutical, bio-pharmaceutical, and radiopharmaceutical facilities worldwide. In an increasingly globalized pharmaceutical industry, cooperation and harmonization have become the norm, driving the demand for continuous monitoring in these facilities as well as the drive for digital solutions to meet your Contamination Control Strategy. In this […]

Semiconductor manufacturing is a highly intricate process, comprising numerous steps and stages, with each one presenting the potential for contamination. Contamination in this context can be particularly costly, leading to yield losses and time wastage. To address these challenges, Real-Time Monitoring Systems offer a powerful solution, providing immediate detection and enabling rapid mitigation in potential […]
Compliance with USP 788 standards doesn’t have to be a complex and laborious process. Recent advancements in liquid sampling technology have made achieving USP 788 compliance both simple and effective. Liquid sampling plays a crucial role in contamination control strategies, ensuring that particulate contaminants are kept out of injectable pharmaceutical products and, ultimately, the bodies of end-users. […]
The Vertex50: A Brief Introduction The Vertex50 is a liquid particle counter equipped with multiple size channels, including nanometers. It has undergone a meticulous testing process to achieve verification, ensuring its reliability and accuracy. One of its standout features is its ability to provide online, real-time monitoring, a critical tool in reducing yield loss in […]
Water quality plays a critical role in the pharmaceutical industry, where stringent requirements must be met to ensure the safety and efficacy of pharmaceutical products. Different pharmaceutical processes demand varying levels of water quality, making it essential to have precise control over water purification systems. Contaminated water can jeopardize product quality and, ultimately, patient safety. […]
Pharmaceutical injectable aseptic injections are essential medical products that are administered through injections, directly into the body. The manufacturing process for these injectable drugs is a meticulous endeavor, requiring stringent control measures to ensure they remain free from any microbial contamination or particles that could compromise patient safety. In this article, we’ll delve into the intricate process […]
In the meticulous realm of pharmaceuticals and controlled environments, precision is paramount. Particle counters emerge as indispensable tools in maintaining stringent quality standards. This blog elucidates the critical role of particle counters in cleanroom certification, routine monitoring, and HEPA filter testing while adhering to ISO standards. Certifying Cleanrooms using Particle Counters as per ISO 14644-1:2015 […]
In the complex landscape of pharmaceutical manufacturing, where the safety and efficacy of medicinal products are paramount, Annex 1 of the EU Guidelines for Good Manufacturing Practice (GMP) for Medicinal Products 2022 stands as a crucial document. One of its core components, the Contamination Control Strategy (CCS), plays a pivotal role in ensuring the production […]
In our rapidly evolving world, environmental monitoring has become a critical aspect of safeguarding our planet’s health. From air and water quality to soil conditions and climate patterns, the data collected through environmental monitoring systems provide valuable insights that guide policies, research, and decision-making. However, the accuracy of these measurements is paramount, and that’s where […]
In 2022, GMP Annex 1 was revised to reflect the changing landscape of technology, discoveries, best practices, and needs. At the time of publication, this is the first and only, but far from the last, regulatory body to make a large update in several years. We do expect to see similar guidelines from the FDA […]
“Risk mitigation” is one of our favorite phrases. The best way to prevent yield loss, ensure end user safety, and high quality is to avoid risk from the beginning. What exactly poses a risk to aseptic manufacturing? First and foremost, contamination. Next, human error and faulty equipment. All combined, these can be a recipe for […]
Environmental Monitoring Systems (EMSs) are important and hefty investments that require typically large amounts of infrastructure; however, modern EMSs are becoming nimble, adaptable, and slimmer. There are so many options and factors that go into making this decision, so where exactly should you start? Here at LWS, we specialize in helping manufacturers make educated decisions […]
Aseptic manufacturing is one of the most difficult forms of manufacturing. From the processes to the inspections to the documentation, this is not an easy task. The right Environmental Monitoring System (EMS), though, can make it simpler. An EMS is made up of a system of filters and sensors that connect to a processing software. […]

Semiconductors are the backbone of our world and the technology that runs our lives, businesses, health systems, entertainment, and more. They do an incredible number of often unappreciated tasks from conducting electricity to processing information. While we have semiconductors to thank for so much in our lives, for them to function in the cell phones […]
Choosing a particle counter for semiconductor manufacturing is not a small decision. It is a massive investment that has ramifications throughout your systems and cleanroom. When you choose one, you should consider all these implications, as well as the particle counter’s functionality in your cleanroom. As clean air and particle counter experts, we’ve compiled a […]
One of the most important things you can do during semiconductor manufacturing is practice proper risk mitigation to reduce contamination. Contamination puts semiconductors at serious risk. It can cause them to underperform, consume too much energy, malfunction, or completely fail. This can result in profit loss, recalls, customer complaints, and damaged reputation. The good news […]
Quality control in semiconductor manufacturing is superficially simple: create consistent quality products. That statement is easy enough to say, but incredibly difficult to truly implement. Quality control involves an in depth understanding of the products you are designing, the processes you have in place, and what can go wrong. In a cleanroom, there are added […]
Lighthouse Worldwide Solutions, a renowned global leader in the manufacturing of cutting-edge contamination control equipment, is proud to announce the opening of its newest facility in Milton Park, Oxfordshire, United Kingdom. This expansion marks a significant milestone for the company as it aims to bring its state-of-the-art technology and expertise closer to its UK-based clients. […]
We talk a lot about life cycles, but have you used this approach to implementing a new system, especially a large one, such as Real Time Monitoring System (RTMS)? If you are familiar with GAMP5, you probably have used a life cycle approach or at least considered it. The 5th edition of Good Automated Manufacturing […]

GAMP5: another day, another acronym. So let’s break it down! GAMP5 stands for the Good Automated Manufacturing Practices 5th version. This publication is monitored by the GAMP committee of the International Society for Pharmaceutical Engineering (ISPE), who also publishes the GAMP guidelines. GAMP5 outlines best practices for computerized system validation in the pharmaceutical industry; however, […]
Good Automated Manufacturing Practices (GAMP) has been defining IT validation since 1991. Since then, it has gone through 5 revisions, the most recent of which was in 2008. This version of the guidelines is called GAMP5. It is published by the International Society for Pharmaceutical Engineering (ISPE), specifically for pharmaceutical cleanrooms. GAMP is both a […]
GAMP5 refers to the fifth publication of Good Automated Manufacturing Practices published by the International Society for Pharmaceutical Engineering (ISPE). GAMP5 is not a set of regulations, but instead a collection of guidelines developed through evidence-based decision making that reinforces the standards set by many regulations, including Annex 11 and 21CFR11. These guidelines outline a […]
At this point, we are no stranger to global health crises. But these crises extend outside of pandemics to regular everyday access to quality healthcare, as well. Standardization of care following evidence-based best practices across the globe improves the world population’s health. Pharmacopeias work across borders to collaborate on best practices and knowledge, improve access […]
In the wake of Pharma 4.0 – the term coined by ISPE to identify the fourth industrial revolution relationship with the pharmaceutical industry – it is critical to look at all elements of an industry for areas of advancement. One of the pillars of standards for the pharmaceutical industry is the United States Pharmacopeia. So […]


The United States Pharmacopeia – National Formulary (USP-NF) is comprised of 6,800 monographs for over-the-counter and prescription products, medical devices, supplements, and other related particles and it is a resource for every step along the drug development process. There are four stages to drug development: R&D, regulatory review, manufacturing and distribution, and medication use. This […]
The United States Pharmacopeia – National Formulary (USP-NF) serves as a resource for drug manufacturers, researchers, and anyone else involved in the development of pharmaceuticals with quality standards for ingredients and manufacturing processes. The USP-NF itself contains over 6,800 monographs for over-the-counter and prescription products, medical devices, supplements, and other related particles. It is revised […]

The article is mainly focused on the impact, causes and reduction of particle generation during the manufacturing of Li-ion cells. Therefore, it is sub-divided into three sections as each aspect is equally important to study the topic in detail. Sections are listed below. 1. Effect on the cell 2. Sources of generation 3. Mitigation techniques We are going […]
Every revolution is, well, revolutionary. Processes and people massively change in a way that rocks the world to its core, in a way that makes it so the world is never the same again. We have witnessed these sorts of transcending changes throughout the last three industrial revolutions and, now, we are arguably experiencing the […]
ISPE’s Pharma 4.0 initiative points to everything the pharmaceutical industry could be if it embraced the same advancements of the fourth industrial revolution: digitization, empowerment, and automation. This is achieved through the integration of Industry 4.0 technology, originally spurred by the internet and now encompassing artificial intelligence, advanced robotics, and automation. Pharma 4.0 is sometimes […]
For years now, robots and automation have been making their way into pharmaceutical production. Lately, though, the power of Artificial Intelligence (AI) has taken the world by storm in the form of ChatGPT: a uniquely human AI bot that will have conversations with you, research for you, and adapt to what you ask it to […]

A lot has changed since the 1700s in the whole world, but especially in the pharmaceutical industry. Over the past 250 years, four industrial revolutions have swept the world, drastically changing the way we manufacture items and their availability – including pharmaceuticals. Industry 1.0 The first industrial revolution spanned from 1760 to 1830, with its […]
Your Contamination Control Strategy (CCS) is the foundation of your cleanroom. This is where you plan how to avoid contamination, what to do in the event there is a breach, and prove that you have done your homework. For too many years, a CCS has been an afterthought. The cleanroom was developed, protocols put in […]
The European Union (EU) Good Manufacturing Practice (GMP) for Medicinal Products for Human and Veterinary Use – Annex 1, commonly referred to as GMP Annex 1, released a new update in 2022. At the time of publishing, we have not yet seen updates in the United States, but should expect to see similar guidelines published […]
We talk a lot about data integrity, but what happens, in the real world, when US Food and Drug Administration (FDA) data integrity protocols are not met? In December, we got to see this scenario play out. Between November 22, 2022 and December 2, 2022, FDA inspectors visited a large pharmaceutical manufacturer’s facilities in India […]
It’s not a secret: PPE is important in every healthcare setting. But in the intensity of a hospital setting, amidst staffing shortages and sky-high hospitalization rates, proper PPE protocols might not always be followed. While trying to save lives and stay afloat in the overwhelm, healthcare workers might slip up. They theoretically know and understand […]
Do you remember the last time you went to a show where there was a “splash zone”? Maybe it was a nautical show with seals or an art exhibit or concert. When you’re seated in a splash zone, you usually are supposed to wear some kind of poncho to serve as a barrier between you […]
No one goes into medicine to cause harm. Doctors, nurses, techs, administrators, PAs, CNAs, and all other staff come together to bring life and give hope. Hospitals should be places of healing – not new illnesses. But for many patients, that’s the unfortunate reality. Hospital Acquired Infections (HAIs) present threats to patients, staff, and the […]
Did you know that approximately 1 out of every 31 hospital patients in the U.S. are expected to contract a Healthcare-Associated Infection (HAI)? That’s right – the Centers for Disease Control and Prevention (CDC) reported that this alarming number of patients contract an HAI every year, totaling almost 2 billion nosocomial infections and 90,000 deaths […]


USP <800> provides guidelines that “describes requirements including responsibilities of personnel handling hazardous drugs; facility and engineering controls; procedures for deactivating, decontaminating and cleaning; spill control; and documentation.” These standards are applicable to anyone in healthcare who is responsible for receiving, preparing, administering, or transporting hazardous drugs. This guideline follows the National Institute for Occupational […]
Our furry family members sometimes go down for the count and need some medication to help pick them up. But what happens when they won’t take a pill? Or need specialized medication? Or have to take multiple medications? The answer for you and your pet might be compounded drugs! Compounded medications are prescribed by your […]
Whether you are a new compounding pharmacist or have been around for a while, you know that compound pharmacies are unique. In your workspace, you have to gown and operate under strict contamination control protocols, but do you know why? As the world’s clean air experts, we’re here to help explain exactly what’s going on […]
You probably don’t think of pharmacies as cleanrooms or controlled environments, but that’s because you are most likely thinking of community pharmacies! Community pharmacies are generally where generic prescriptions are sent and are readily available throughout the United States. There are roughly 56,000 community pharmacies in the states, but only 7,500 of those specialize in […]
In 2020, a vote found EN ISO 14698 no longer suitable for use in Europe, and, thus, it was replaced by EN 17141. Now, there is a push to accept EN 17141 as the international standard, as it is significantly more up to date and applicable than EN ISO 14698. EN ISO 14698 was last […]
After 17 years of no revisions, a formal vote in 2019 replaced EN ISO 14698 with EN 17141 in Europe. Shortly after, confusion ensued. While CEN/TC243 (the technical committee in CEN) had found EN ISO 14698 obsolete and replaced it with a new guidance (under a new number, to avoid confusion), old versions still existed […]
You will hopefully not be shocked to learn that data integrity is an integral part of a properly running cleanroom. If this does come as a surprise to you, we highly recommend you read on and take notes. But for many cleanroom or particle counter professionals, the term “data integrity” has been drilled into our […]
At the start of 2022, we outlined some trends we expected to see grow in popularity and necessity throughout the year. They included paperless cleanrooms, advanced self diagnostics, multiple transfer options, and smart batteries. Now, as 2022 comes to a close, we want to consider how these trends have played out in the last year […]

Have you ever heard the old wives’ tale that if you water your garden at night, mushrooms will grow by morning? While there’s some truth to this timing, because mushrooms grow when there’s a certain moisture content and the night prevents water from evaporating at the same rate, there is a deeper, hidden, much more […]
During the 2020 fire season, more than 106 large wildfires occurred in the states of Oregon and California. The fires were battled by tens of thousands of wildland firefighters, but still burned more than 6.7 million acres. While thousands of people lost their homes, almost every resident of the state was able to see the […]
EN 17141 Cleanrooms and controlled environments – Biocontamination control is a relatively new European standard, introduced in 2020, that establishes and demonstrates best practices to control airborne and surface microbiological contamination in controlled environments, such as cleanrooms. This standard applies specifically to European cleanrooms in the pharmaceutical and biopharmaceutical, hospital, and food industries, as identified […]
An airborne particle counter only needs to know two things to begin sampling: where it is and how long to sample. From there, it should be built around ease of use and reliability. You should be able to trust that your airborne particle counter is working for you and not the other way around. You […]

What is a foundational piece of your cleanroom contamination control strategy? Your gowning protocol. Humans, by far, produce the most particles in a cleanroom and your gowning protocol can limit that number. While we have a number of ways to improve your gowning protocol across the board, it is important to recognize that different ISO classifications require […]
An active air sampler is one of the tools you can use in your cleanroom to monitor contamination, but, specifically, it is the tool you can use to monitor viable contamination. These are live microbes, so they have the potential to grow, like mold or fungi. While a particle counter can tell if contamination is […]

This year, Lighthouse Worldwide Solutions is celebrating its 40th anniversary. Over the last 40 years, we have seen massive changes in particle counters and cleanroom monitoring technology intertwined with advances in technology outside the cleanroom. Today, we’re looking back at where it all started and the influences on the industry that have progressed it to […]

In 2017, GMP released a draft update to Annex 1, which received a lot of feedback. Now, GMP has released a finalized version of the updated Annex 1, which addresses many of the concerns and questions raised about the draft version. The largest changes and shifts in this document are found in the increased focus […]
A cleanroom classification basically tells you how clean a cleanroom is. While we typically consider cleanrooms to use HEPA filters and multiple layers of protection, cleanrooms can really be any room where precautions are taken to ensure that the product stays clean from contaminants. But if that’s the case, then what makes one cleanroom stand […]
The European Union (EU) Good Manufacturing Practice (GMP) for Medicinal Products for Human and Veterinary Use – Annex 1, commonly referred to as GMP Annex 1, was originally published as a draft in 2017. This draft left many people operating in the pharmaceutical industry wondering at some of the proposed changes to the regulations that […]

Testing of compressed gases is a GMP requirement when such gases are used in cleanroom applications. It is critical for such testing to occur since product process zones in ISO 5 cleanrooms and zones require tight control on contamination and compressed gases used may also if not checked correctly be an avenue for particulate contamination […]
It is no mystery that cleanrooms need to be, well, clean. From the surfaces and tools to the air in the cleanroom, everything needs to remain at a certain level of cleanliness depending on the cleanroom’s classification and application. Those criteria extend to the compressed gasses used in cleanrooms, as well, like oxygen, hydrogen, methane, […]
Qualification of Vertex50 to measure and detect ≥50nm trends in UPW systems The Vertex50 has TRUE sensitivity at 50nm has been qualified to measure and detect ≥50nm particles in Ultra-Pure Water Systems (UPW). UPW systems are used in many industries worldwide to provide high purity water. Ultrapure water is a commonly used term in the […]
Lighthouse offers an opportunity to sample the contamination level in compressed gases. In the electronics industry as well as the Pharma industry contamination control of compressed gas has been getting more important over the last few years.
If you use compressed gasses in your cleanroom, ISO 8573 is an important set of guidelines and regulations for your cleanroom, specifically ISO 8573-1:2010. This set of guidelines is maintained and governed by the International Organization for Standardization (ISO), an international, non-governmental organization dedicated to creating worldwide standards for a variety of industries. ISO standards […]
Good Automated Manufacturing Practices (GAMP). GAMP is both a technical subcommittee of the International Society for Pharmaceutical Engineering (ISPE) and a set of guidelines for manufacturers and users of automated systems in the pharmaceutical industry. ISPE is the world’s largest not-for-profit association serving its members by leading scientific, technical and regulatory advancement throughout the entire […]

If you know us, then you know that clean air and water is our passion in life. But what happens when you cannot prove to the regulatory authorities that your air and water is clean? Cleanrooms and monitoring systems generate a lot of data. In an 8 hour shift, as many as 5,000 individual count […]

With the never ending shift towards continuous quality improvements within the manufacturing of pharmaceutical products it is worth looking at the current requirement of GMP and also 21CFR11 in the context of GAMP 5 requirements. How a Company creates, maintains, retrieves, corrects and controls data can affect product quality. How a company reacts to out […]
Welcome to the age of the internet! Pharma 4.0 is the International Society for Pharmaceutical Engineering’s (ISPE) version of Industry 4.0, also called Smart Factory. Essentially, Industry 4.0 is the fourth industrial revolution. It encapsulates the changes we are seeing happen in the world because of the internet. Pharma 4.0 is ISPE’s roadmap for pharmaceutical companies to also […]
A PQ is a performance qualification which is conducted prior to a system becoming operational. The PQ tests the system to ensure it meets its operational objectives in a real world operational environment. It is the final step in equipment and systems qualification. A PQ should never be a re-execution of the vendor supplied Operational […]
Did you know we start to mitigate risk in our lives as early as 6 months old? That’s right: at 6 months old, an infant can recognize a stranger and knows – in their gut – that the unfamiliar person could pose a risk. The following cries are the baby’s form of risk mitigation. But […]
What does your dream home look like? Maybe it’s a beautiful home way up in the mountains, where the air is fresh and the land is quiet. You sit on your porch, enjoying a hot coffee as the weather turns brisk… And the first snowflake of the year falls. How exciting! This is your first […]

If you are relatively new to the cleanroom world, you’ve probably heard the word “reverse osmosis” thrown around a few times. You might have also heard it used in marketing and advertising of different purified water brands. What does it bring to mind? For many of us, a phrase we learned long ago in high […]
We often throw around the word “contamination” and how dangerous it can be in a cleanroom. And if you work in or around cleanrooms, you know how true this is. But to the everyday consumer, products being free from contamination is often taken for granted. We often don’t even think about contamination control! For many, […]
If you are working with a pharmaceutical or semiconductor manufacturing cleanroom, you most likely have seen ultrapure water in action. This water is used for water for injectables (WFI) in pharmaceuticals and as a chisel for semiconductors. While it has many other purposes, these are the primary two. And those two industries use a lot […]
Anyone who has used an N95 face mask knows that they are far from comfortable: they pinch in all the wrong places, they fail fit tests, and they limit your vision. As you have to fiddle with your face mask, its functionality and protection comes into question. As the world’s leading clean air experts for […]

If you have ever had a shot or used a cellphone, you have relied on a liquid particle counter! Liquid particle counters are used to measure the size and volume of particles in a sample of ultrapure water used in cleanrooms. Ultrapure water is water that has been specially processed to minimize contamination. It is […]
ISO 14644 is a standard for cleanrooms that was developed by the International Standards for Organization (ISO). The ISO has been in existence since 1947, after 65 delegates from 25 different countries met to discuss its future the previous year. They saw the need for a non-governmental body whose sole job it was to determine […]
What is the biggest villain in the contamination control and cleanroom world? Particles. Contamination. This contamination can cause yield and financial loss, delays, frustration, and physical threats to the end user. It is something that should be avoided wherever possible. To minimize contamination risk in your cleanroom, you first need to understand what contamination control […]

Is there a way that you can improve response times, decrease contamination delays, and reduce personnel frustration in your cleanroom? There absolutely is. The answer is a real time monitoring system. These are systems that monitor your cleanroom for particle contamination in real time. They provide continuous monitoring, alarms, and feedback for your team. The […]

The following questions will help you ask the right questions when implementing a system thatadheres to 21CFR Part 11. It is important to satisfy the requirements of this FDA code in order toenable Data Integrity and Data Traceability as well as the electronic signatures that verify the dataas original, valid, and accurate
Cleanroom maintenance is the key to ensuring your cleanroom continues to operate at peak form. Failure to conduct regular maintenance can have devastating consequences for your cleanroom. For instance, in 2018, a NASA cleanroom was contaminated with fungi. While the fungi was found around the cleanroom, it was heavily concentrated around a filter that had been installed […]
21 CFR Part 11 outlines the federal requirements that help to ensure that electronic records are trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper. 21 CFR Part 11 has 10 chapters over 3 subsections. These subsections are General Provisions, Electronic Records, and Electronic Signatures.
At the end of the day, in a cleanroom: time is money. Cleanrooms are expensive to operate and, while they are running, they have a lot to do. Technicians gown up and put their expertise to work on various applications. But due to the nature of cleanrooms, SOPs and regulations must be minded. So there […]
Air Sampler impaction technology has been around for decades. However the d50 is a little know aspect of air sampler technology design and it plays a critical role in air sample capture. Aerosol Impaction is the process in which particles are removed from an air stream by forcing the gases to make a sharp bend. […]
Vaporized Hydrogen Peroxide (VHP) is a powerful chemical used to sterilize surfaces andequipment in the cleanroom, specifically in the medical equipment and pharmaceuticalindustries. It can cause deterioration and breakdown of equipment. Due to the nature of theindustries in which it is used, VHP is often used to sterilize equipment while the cleanroomis in use. Thus, […]
The Good Automated Manufacturing Practices (GAMP) were developed by the International Society for Pharmaceutical Engineering (ISPE) for pharmaceutical cleanrooms. The ISPE remains the governing body for GAMP. According to ISPE, GAMP is, “A system for producing quality equipment using the concept of prospective validation following a life cycle model. Specifically designed to aid suppliers and users […]
A cleanroom is a specifically designed room that controls contamination. Cleanrooms are used in practically every industry where small particles can adversely affect the manufacturing process and product. The main components of a cleanroom are what keeps the cleanroom clean. To control contamination in a cleanroom clean air is required, access is limited and outside […]

How many times have you heard us say, “your cleanroom filtration is critical”? Probably more than once if you’ve spent any time interacting with us. But not all filtration is the same! Depending on your filter and application, there are different cleanroom particle filtration techniques. Today, we’ll be covering 4 of them. Straining/Sieving This technique is […]
We talk a lot about contamination control strategies in a cleanroom, but there is another type of strategy you need to consider: air distribution. This strategy is typically put into place during the planning and building of a cleanroom because it has a lot to do with infrastructure. But understanding your cleanroom’s air distribution strategy […]
Your filters are what separates your cleanroom from just some ordinary, run-of-the-mill room. They stop potentially harmful contaminants from entering the room. They prevent yield loss and harm to the end-user. Most importantly, the filters’ MPPS defines your cleanroom’s potential classification. This classification limits your cleanroom’s applications. Cleanroom filtration is defined by the type of […]
Cleanrooms require air filters in order to trap contaminants from entering the cleanrooms air supply through its HVAC system. Cleanrooms are controlled environments where the control of temperature/humidity, pressure and particles are essential for optimum operational performance.

When you need to classify your cleanroom, do you reach for your trusty photometer or particle counter? Both photometers and particle counters are devices used in cleanrooms to measure particles, but they go about it in different ways and report different data. According to ISO 14644, both are acceptable forms of testing for classification, especially […]
Particle Counter management is a critical process in ensuring particle counter health and data integrity. The accuracy of the data from particle counters is critical in regulated industries. Particle Counters should be calibrated regularly based on their use. Manufacturers recommend that Particle Counters are calibrated at a minimum on an annual basis.
What does a perfect cleanroom look like? Zero contamination, immaculately gowned personnel, and no malfunctioning equipment, ever. That’s the dream, right? While perfection might not be attainable, we can work to get as close as possible to it. Not only will operating your cleanroom by these “nearly perfect” standards keep your data in line for […]

Does it matter what kind of particles exist in your cleanroom? Isn’t all contamination bad? On the surface, yes. You just need to know if your cleanroom is contaminated or not. Any contamination poses a risk to the contents of the cleanroom and, in some industries, a threat to the end-user or product. But we […]
In an ever changing world, we are constantly seeing new regulations and expectations. This is especially true in the area of computerized system compliance and validation. GAMP was designed to address these needs. GAMP (Good Automated Manufacturing Practices) is a system designed specifically for suppliers and users in pharmaceuticals. GAMP certifies a system that produces quality […]

Cleanroom Certification is a process of validation. This validation process certifies that the Cleanroom operating conditions meet the intended design parameters. Room Particle Counts are tested using a Portable Particle Counter sampling at a defined volume at evenly spaced test locations around the cleanroom based on current Cleanroom Standards. The most accepted and widely used […]
So you’re going to classify your cleanroom. But what cleanroom classifying tools can you use that will make the process painful and easy? First things first, let’s define cleanroom classification. The International Standards for Organization (ISO) is one organization that sets cleanroom classifications. ISO is a non-governmental agency that sets standards in a number of industries. These […]
So what exactly makes a cleanroom… A cleanroom? First and foremost, a cleanroom needs to be clean. You are able to communicate just how clean it is through its cleanroom classification. But you cannot attain the classification without first making sure your cleanroom is really that clean. You do this through a proper cleanroom contamination control strategy […]


Water is one of the major utilities used by the pharmaceutical industry. Different grades of water quality are required depending on the different pharmaceutical uses. Control of the quality of water, in particular the microbiological quality, is a major concern and the pharmaceutical industry devotes considerable resources to the development and maintenance of water purification […]
What happens if an injectable is contaminated? Infections, complications, vein irritation, local tissue infarction, anaphylactic shock, other health risks, and potentially death… The end-user is put in jeopardy. That is why it is vital we prevent contamination at the source: in the cleanroom during the manufacturing of the pharmaceuticals. This involves utilizing a liquid particle counter […]
Water is an essential part of everyday life, and certain cleanrooms are no different – especially in pharmaceuticals. But water is unique apart from other products and process ingredients because ultrapure water is not subject to testing or batch-lot release before use and is drawn from on-demand systems. Additionally, results from water testing are not […]
Water. It makes up 70% of our bodies, but, even more importantly, it serves as a vital ingredient and manufacturing component in cleanrooms. Before we are able to introduce it into the cleanroom setting – especially as part of the product, such as in injectables and other pharmaceuticals – it needs to be purified. We’ve […]
We are all too well aware of the adverse effects of particulate matter contamination within parenteral injectable pharmaceutical products and the consequences for patient safety. Here, particulate matter refers to the small, sub visible particles. The United States Pharmacopoeia, USP <788> provides two tests for detecting such particulates: light obscuration and microscopic assay. Both are […]
As the world’s clean air experts, we talk a lot about monitoring your cleanroom’s cleanliness by keeping an eye on airborne particles. But what happens when airborne particles are no longer airborne? That is what we call particle deposition. When a particle deposits, it causes contamination and chaos in a cleanroom. So it is important […]
Everyone with a cleanroom could use a particle counter, right? But which particle counter is right for you, and WHY is that particle counter a portable one? First and foremost, a portable particle counter creates its own vacuum, can be moved, and typically displays its reading on a built-in screen. This prevents you from leaving […]
An Environmental Monitoring System (EMS) is very different from a Building Management System (BMS). A building management system (BMS), also known as a building automation system (BAS), is a computer-based control system installed in buildings that controls and monitors the building’s mechanical and electrical equipment such as ventilation, lighting, power systems, fire systems, and security […]
How It’s Made: Electric Vehicle Batteries Myth or truth: electric vehicle batteries are just as bad for the environment as driving a gas-powered car. Do you have your answer? Do you think it’s a myth or the truth? It’s a myth! This myth comes from the fact that electric vehicles have large batteries that can […]


Your Environmental Monitoring System (EMS): the frontline between your product and contamination. Or, at least, letting you know there is contamination so you can determine what the problem is and fix it! This means that an effective EMS is able to recognize when things are going wrong. The cornerstone of this process is conducting a Performance […]
Your Environmental Monitoring System stands between you and system failure, product recalls, contamination, and potential lawsuits. Sounds intense? We think so, too. But as the world’s clean air experts, we know it can be done right so at the end of the day you save time, money, and stress. Today, we’ve outlined the steps you […]

Imagine you’ve been rear-ended. There’s a little dent in your fender, but the paint has been damaged. When you go to get it fixed, what will matter more to you? The exact specifications of the process used to repair the dent or the guarantee that the paint will match your car’s paint job? Probably the […]

Does it matter what kind of particle exists in your cleanroom? Yes, it does! Two types of contamination include viable and nonviable particles. Depending on which is present in your cleanroom, you will be able to determine where your cleanroom contamination control strategy is suffering and how to build a cleaner cleanroom. To determine the […]
When we think of cleanrooms, we usually think of manufacturing, technology, food processing, research, etc. But have you considered how those processes are used by NASA for space exploration? That’s right! NASA uses cleanrooms – and quite often, too! Not only is their equipment highly tuned, so it needs to be manufactured in a clean […]


In many industries, face masks have played a significant role for many years! Surgeons wear them in surgeries. Scientists wear them in cleanrooms. Construction workers wear them on the job. And many more! But which face mask is best for which job? Some offer filtration from very small particles (viruses and bacteria), while others only […]
What is something that is truly indestructible? Comic book fans will say a vibranium-metal alloy, while others might say that diamonds are about as close as we’ll get in reality. But something is being manufactured on our planet today that is genuinely indestructible. Plastic. That’s right: the plastic we are making today is here to […]
Your particle counter is a foundational part of your cleanroom contamination control strategy, as it stands between you and contamination notification. But your particle counter has a wide array of electronics that should be monitored to ensure the reported data is accurate. There are a few things you can look for in your particle counter […]


It’s no secret that COVID-19 has drastically impacted the world around us. Throughout August 2021, the illness has overrun hospitals. To combat the spread of COVID-19 amongst hospital staff and patients, hospitals have been paying extra attention to their contamination control procedures. Aside from HEPA filters, hospitals have been using a piece of cleanroom technology to […]
Southern Oregon Air Quality: Introducing Rogue Valley Breathe Easy Here in Southern Oregon, air quality is a common topic of conversation. During the summer, we are plagued by smoke from wildfires. Since smoke is able to travel so far, it doesn’t matter if the fire is in Northern California, Western Oregon, or somewhere else in […]

Cleanrooms are just that: clean. Therefore, there are strict requirements around what is allowed in a cleanroom and what is not. While exact guidelines might change in different cleanrooms, the following are considered general guidelines and best practices about what should not be allowed in a cleanroom. Street Clothes Your street clothes carry billions of […]
Looking for an affordable alternative to a permanent cleanroom? Portable cleanrooms (also called modular cleanrooms) are usually constructed with a rigid frame such as steel and have panels made from plastic, vinyl or clear flat materials. They offer many of the benefits of a permanent cleanroom, but with the added versatility of being movable and […]
Air particles are measured by forcing air through a cavity in a particle counter which uses a laser to measure and count the particles. This is done through a process called light scattering. Parts Of A Particle Counter Inside a particle counter, you will find a laser sensor block. This is where the particles are sized […]
Features to look for in your particle monitor to ensure compliance of your cleanroom Environmental monitoring lies at the heart of regulatory compliance for cleanrooms used in pharmaceutical, bio-pharmaceutical, and healthcare facilities. Facility managers need to understand what components are needed for a monitoring program, but they must also ensure the data they produce is […]
Good Manufacturing Practices (GMPs – sometimes referred to as Current Good Manufacturing Practices or CGMPs) are federal regulations set by the FDA to cover how pharmaceuticals and food products are manufactured. They are guidelines taken for granted by many consumers – they assume what they put in their body will have been safely produced – but […]

Light scattering is a technology that revolutionized the airborne particle counter industry, because it is able to quickly, efficiently, and accurately determine the size and number of particles that pass through an airborne particle counter in real time. Today, it is used in the airborne particle counters that set industry standards, because it is the […]



Your cleanroom contamination control strategy is the frontline between you and contaminated products, which can lead to yield loss, slowed production, and possible reputation damage. So when was the last time you really dove into your cleanroom contamination strategy? We highly recommend reviewing your strategy yearly to make sure you are still meeting industry best […]
Are you considering a new particle counter for your cleanroom? There are a number of options that might be right for you. Before reading through the pros and cons of each particle counter, we recommend you create a list of what you are looking for in your particle counter. On this list, we recommend you […]

Did you know that water might be clean enough to drink but not clean enough to use in your food? When it comes to water used in a cleanroom and manufacturing setting, it’s critical that the water is free of all contaminants and particles to maintain consumer safety. But what industries exactly need an ultrapure […]
What Is Cleanroom Paper? [Case Study] Do you use print-outs in your cleanroom? What kind of paper do you use? Could we be doing better than current industry standards? We think so. This is why we’ve started to investigate “cleanroom paper” and how this paper can impact the number of particles in a cleanroom while […]

Water is an extremely powerful force. We see it carve its way through land to form amazing geographical features like the Grand Canyon. So what can water do in your system? Ultrapure water systems clean water – keeping it particle free – so it can be used to clean and etch in manufacturing, especially in […]
Contamination in pharmaceuticals can quickly turn into a nightmare with yield loss, recalls, bad press, and harmed consumers. Thus, it’s critical to stop contamination from ever being an issue during production in your cleanroom. Here at Lighthouse Worldwide Solutions – the world’s clean air experts – we’re pros at keeping cleanrooms extra clean. It’s our […]


As clean air experts, we talk a lot about making sure that the air in a cleanroom stays as clean as possible. We do this to meet Food and Drug Administration (FDA) Good Manufacturing Practices (GMPs) in pharmaceuticals and keep our products on the shelves and helping people. When we bring contaminants into a cleanroom, […]

In the COVID-19 era, indoor air quality has become a hot button topic. There are a lot of interesting facts about indoor air quality that you might not know, but one of the most surprising facts is that your indoor air quality was impacting you long before COVID. As the world’s clean air experts, we […]
March is Women’s History month: dedicated to recognizing the critical contributions of women throughout history. As those who work in the STEM community, we are thoroughly thankful for and impressed by the women who have changed the shape of the world – in the past and today. Today, we wanted to take a few moments […]


Handheld particle counters are just what it sounds like: particle counters that you can hold in your hand. They are typically use to spot check a cleanroom or to test air temperature and humidity. Handheld particle counters are a versatile, ergonomic tool that deserves its place in the modern cleanroom, along with indoor air quality […]

If you’re working for a company already using a cleanroom, you’re well aware of the benefits of a cleanroom and how important they are. But if you’re wondering how you can improve your production, then a cleanroom might be the answer. Either way, a cleanroom is not a simple beast. It is a multi-layered room […]
Particle counters do just that: they count particles. They are used in cleanrooms to count and size the particles in the air so the cleanroom can stay in proper working function and meet crucial certification criteria. The fundamentals of particle counters are relatively simple. You need to know how and why they work. If you have a […]
This Whitepaper is intended to assist users that are looking to integrate their ApexZ unit into LMS Pharma’s facility control/monitoring system. This document covers the following topics: 1. Establishing a connection to the ApexZ unit via the Configuration Tool 2. Establishing a connected unit into LMS Pharma’s Database Tree 3. Adding a connected unit to […]













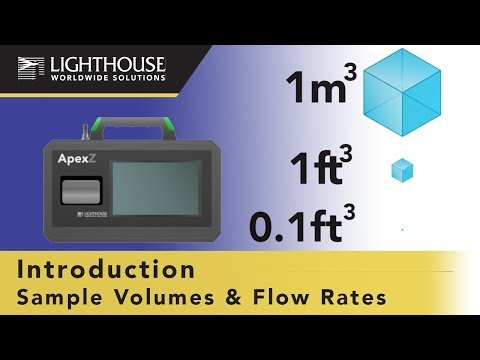













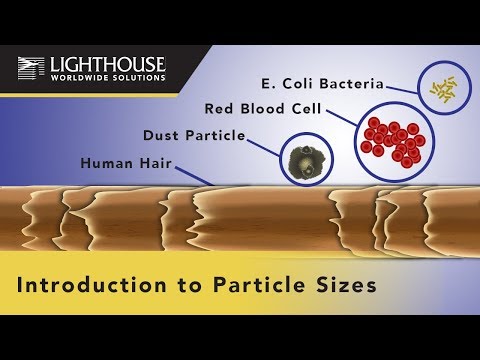


























The new Biotech Training Facility (BTF) is a state-of-the-art training center where students can learn how to produce (bio) pharmaceuticals, under GMP and biosafety conditions, in a realistic environment. The cleanrooms in the BTF 2000m² (37000ft²) consist of gowning rooms, Class A to D grades. The facility is built in the heart of the Dutch […]
Lighthouse Worldwide Solutions attended the Interphex Osaka Japan exhibition on February 24-26, 2016. The AC100 and the new Apex P3 with bigger screen on this exhibition were presented. Both units were very well received by the customers! Also, they trained Kondoh on the AC100 and Lighthouse is ready to sell this unit in Japan! What […]
Abstract This article discusses the mechanisms of particle deposition onto cleanroom surfaces. The main mechanism for particles above 0.5 µm is gravitational settling. Turbulent deposition and electronstatic attraction can also occur at all particle sizes, and for particles below 0.5 µm Brownian diffusion is important.



KNOWLEDGE CENTER
SERVICE & CALIBRATION
SUBSCRIBE
Get the scoop on new products and the latest tech papers, webinars and more…