Welcome to the age of the internet! Pharma 4.0 is the International Society for Pharmaceutical Engineering’s (ISPE) version of Industry 4.0, also called Smart Factory. Essentially, Industry 4.0 is the fourth industrial revolution. It encapsulates the changes we are seeing happen in the world because of the internet.
Pharma 4.0 is ISPE’s roadmap for pharmaceutical companies to also embrace the power of the internet and digitization in its entirety. At the peak of Pharma 4.0, ISPE envisions organizations using the full power of digital technology to provide safe, fast, and effective solutions for the problems we are facing today.
Pharma 4.0 is developed around 12 theses and a 4 part operating plan, which is shared with Industry 4.0.
Pharma 4.0 12 Theses
The following theses were developed by ISPE to help pharmaceutical companies transition into the new digital age. They are directly quoted from ISPE.
- “Pharma 4.0™ extends/describes the Industry 4.0 Operating Model for medicinal products”
- “In difference to common Industry 4.0 approaches, Pharma 4.0™ embeds health regulations best practices.”
- “Pharma 4.0™ breaks silos in organizations by building bridges between industry, regulators and healthcare and all other stakeholders.”
- “For the next Generation Medicinal Products, Pharma 4.0™ is THE enabler and business case.”
- “For the established products, Pharma 4.0™ offers new business cases”
- “Investment calculations for Pharma 4.0™ require innovative approaches for business case calculations.”
- “Prerequisite for Pharma 4.0™ is an established PQS and controlled processes & products.”
- “Pharma 4.0™ is not an IT Project.”
- “The Pharma 4.0™ Operating Model incorporates next to IT also the organizational, cultural, processes & resources aspects.”
- “The Pharma 4.0™ Maturity Model allows aligning the organizations operating model for innovative and established industries, suppliers and contractors to an appropriate desired state.”
- “Pharma 4.0™ is not a must, but a competitive advantage. Missing Pharma 4.0™ might be a business risk.”
- “When moving from blockbusters to niche products and personalized medicines, Pharma 4.0™ offers new ways to look at business cases.”
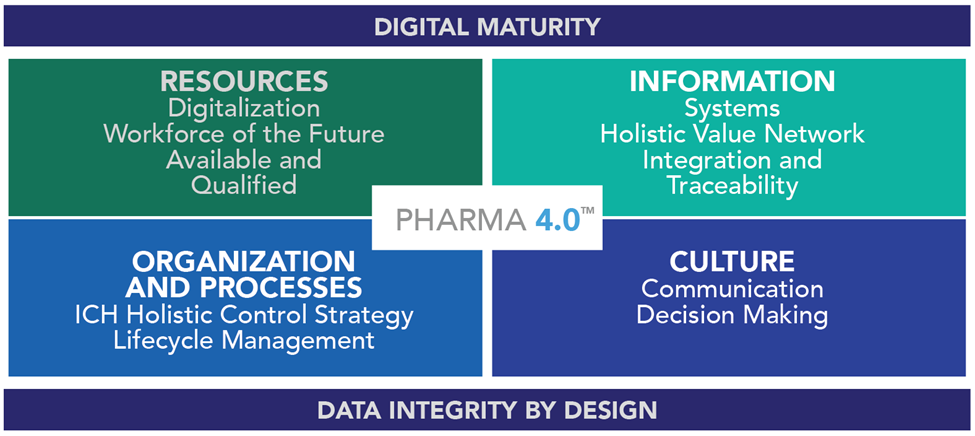
Pharma 4.0 4 Part Operating Model
Pharma 4.0 is a complex and intricate initiative to help pharmaceutical companies transition into a complete digitization era, but it is based on a 4 part operating model, including: resources, information systems, organization and processes, and culture.
Resources
ISPE considers an organization’s tangible assets to be its resources. This comes in the form of its human capital, machinery, tools, equipment, and final product. To adapt to Pharma 4.0, resources need to be flexible and adaptive.
For instance, humans will need to require less control and formalized rules. They are flexible, critical thinkers, and creative. To save them time, many recurring decisions will be automated in Pharma 4.0
To stay flexible, equipment (such as robotics, modular equipment, and 3D printing) should be designed to process a small amount of material easily. This means avoiding extensive setups.
For ultimate flexibility, humans and machines should work together in a hybrid environment. This sort of augmented reality promotes collaboration that will make the humans lives easier and workflow simpler and faster.
Information Systems
Information systems use data provided by people, information, and communication technology. They are socio-technical systems that prepare, process, store, and transfer that data. They provide 3 integrations of data: 1D, 2D, and, with Industry 4.0, 3D.
1D integrations aggregate the data in a decision hierarchy and 2D communicates the information along the value chain. Now, with Industry 4.0, 3D integrations communicate the data across the value chain, as well.
In the past, pharmaceutical organizations have focused on a systems-based architecture. To transition to Industry 4.0 or Pharma 4.0, the organization must shift into a service-oriented design. This is one way the organization can stay agile in the marketplace.
Organization And Processes
This portion of the model is two-fold: a company’s organization refers both to its internal structure and operational process as well as the company’s position in the marketplace. Pharma 4.0 closely involves the organization’s processes. They need to be agile and value-driven. To achieve this, employees should be empowered, informed, and able to make operational decisions without authorization. That is, a business’s hierarchy is more about organization than daily decision making. Through leadership transparency, employees should be able to make decisions in line with interests and needs of the organization.
Culture
Culture describes the soft factors an organization cultivates through their value system and collaboration. While some managers assume they have no control over the culture – that the culture is simply birthed from the people working from the company – this is not entirely true. They must take command of values that the company promotes and shift the culture to be a part of Pharma 4.0.
That is because, in Pharma 4.0, people need to be able to interact with virtual communities. Each of these communities will most likely have their own culture, so the people must be flexible and adaptable for information exchange. This information exchange is empowering and educational. The freedom and empowerment from the close access to information will allow people to make decisions faster and without waiting for authorization unless necessary.
How Can You Stay On Top Of The Industry Changes?
In highly competitive and regulated industries, like pharmaceuticals, it is only expected that changes will come to the industry. It is critical for the survival and growth of your organization that you stay on top of these changes.
The good news is that we’ve made it as easy as possible for you to do just that!
In our Knowledge Center, we have compiled over 40 years of industry knowledge on environmental monitoring, cleanrooms, and clean air and water in general! And we’re giving out access for free.
We break down complex topics into easy-to-understand white papers, blogs, videos, webinars, and more. So you can become the expert in the room and stay up to date on the inventory.


