Water. It makes up 70% of our bodies, but, even more importantly, it serves as a vital ingredient and manufacturing component in cleanrooms. Before we are able to introduce it into the cleanroom setting – especially as part of the product, such as in injectables and other pharmaceuticals – it needs to be purified. We’ve outlined six of the most popular and effective water purification methods used in cleanrooms.
Microporous Filters
A microporous filter is exactly what it sounds like: a filter containing pores of different sizes depending on your requirements. Water is passed through the filter, and large particles are sloughed off.
While this method in and of itself is not sufficient to create clean enough water for injectables, it is considered a vital part of any clean water purification system.
Reverse Osmosis (RO)
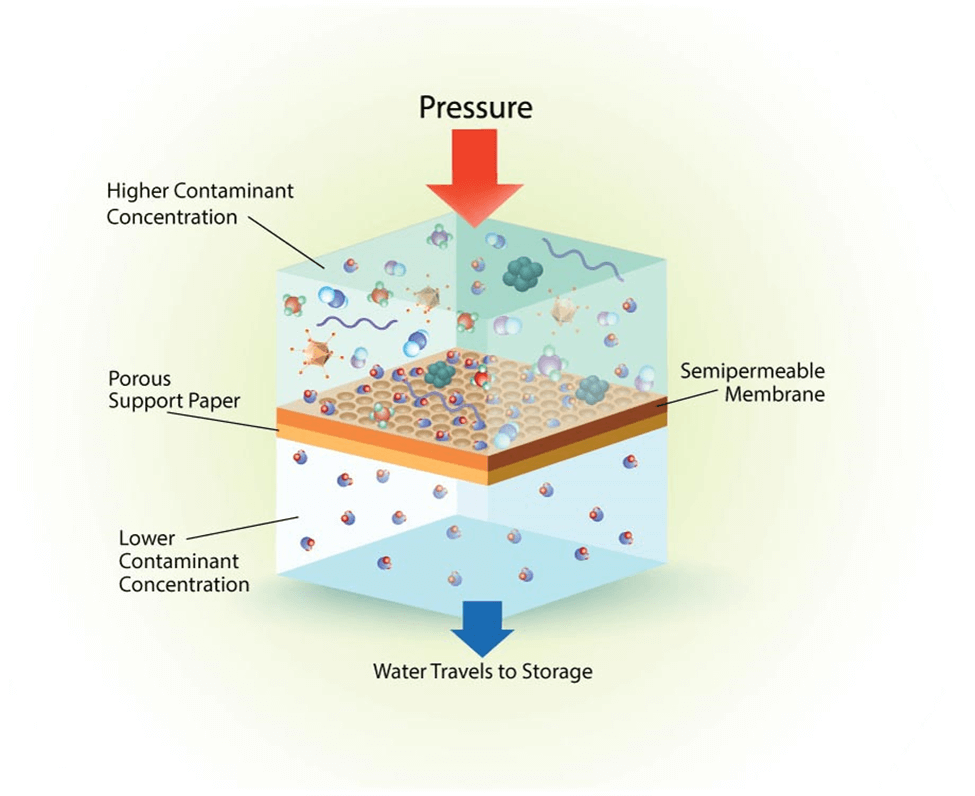
Reverse Osmosis is a powerful water purification method that typically eliminates around 90% to 99% of ionic contamination. It also removes most organic contamination from the water. It does have a drawback, though, as it does not remove non-ionic particles weighing <100 Dalton as well as it sifts out larger particles. It also does not remove dissolved gases. But since it is able to remove so many contaminants, it is considered one of the most cost-effective options. To remove even more contaminants, it is often paired with ion exchange or electro-deionization.
Pretreated water is pumped through a thin film composite membrane under pressure, around 60-220 psi. These membranes are often polyamides. This allows for stability with many different pH levels, but they are susceptible to damage caused by oxidizing agents like chlorine.[3]
Only part of the water makes it through the membrane, though. The ratio of the volume of water that gets through the membrane to the volume of sloughed off feedwater is called the “recovery.” The water that is shedded as part of the recovery is filled with particulates and waste – like organic matter and salts. A recovery of 75% to 90% is considered normal. The higher the quality of the feedwater, the better the recovery will be!
Degassing Membrane (DG)
We often see membrane degassing when the feedwater has a large amount of dissolved carbon dioxide. Why? Because membrane degassing involves allowing water and gas to come close enough to interact but not exchange along a membrane. The carbon dioxide is able to pass through the membrane, removing the CO2 and keeping low conductivity.
Using pressure technologies, we can force the dissolved gases in the water coming into contact with the countering gases at the membrane to enter their own gas state. Then they can be properly eliminated from the water[4] .
Distillation
Compared to the other methods on this list, distillation is considered relatively simple. Water is heated to a temperature that causes it to evaporate into steam, leaving behind all the heavy impurities. Unfortunately, the lighter impurities evaporate with the water into the steam. Thus, the steam must then be passed through a filtration system, as well, to remove these additional contaminants.
Ion Exchange (IX)
Ion Exchange (IX) involves allowing the feedwater to come into contact with a bed of ion exchange resins. In brief, these resins replace ionized particles with H+ and HO- ions.
The ion exchange resins are often in the form of beads. They are typically cationic (usually polysulfonic acid derivatives) or anionic (benzyltrimethyl quaternary ammonium hydroxide or benzyldimethlyethyl quaternary ammonium hydroxide derivatives).
The resin attracts positively charged particles and replaces them with heavier charged cations. To reinvigorate the cation resin, it just needs to be exposed to a large amount of strong acid.
On the other hand, the positively charged resin using anions replaces the negatively charged ions. To bring life back to the anions, you need to expose them to a strong sodium hydroxide solution (NaOH).
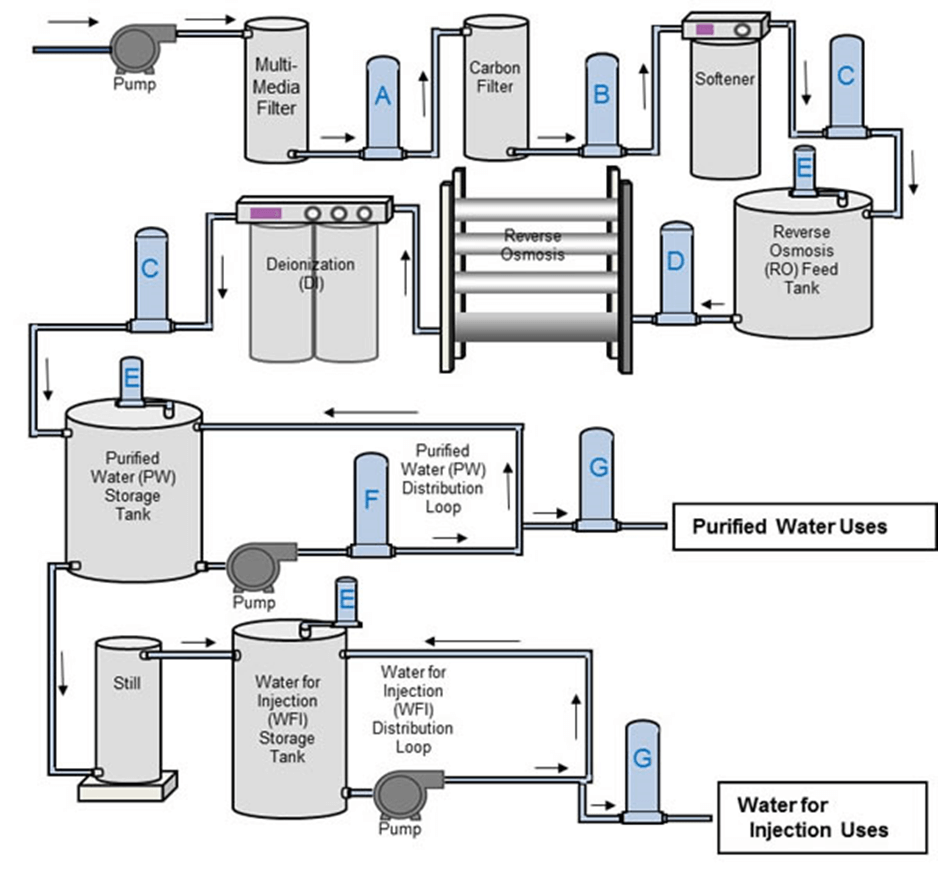
Continuous Electrodeionization (CEDI)
Continuous electrodeionization (CEDI) is a popular purification method in pharmaceuticals for a couple of reasons. First, it does not introduce unnecessary chemicals into the process. Second, it solves the problems presented by traditional and basic ion exchange. Third, it has an incredible success rate in conjunction with a more mechanical process, like RO or DG.
So, where does ion exchange fall short? We typically see the release of the ions as they start to wear out. To overcome this obstacle, continuous electrodeionization was developed.
Feedwater is passed through either the RO membrane or degassing membrane and is then passed into chambers with either anion or cation resins. The big difference is that CEDI uses membranes backed by magnetic forces to pull the ions from the water. So it still uses the resins, but in conjunction with the membranes and multiple chambers, we see the ions wear less.
The CEDI chambers remove roughly 99% of the particles introduced to them. When used in combination with RO, which already removes about 90% of contaminants, we see a really pure end product. The RO process does a phenomenal job of removing bacteria, which reduces the risk of microbial contamination.
The Power Of Liquid Particle Counting
A really solid contamination control strategy involves some combination of water purification techniques to achieve the optimal end product. But your strategy should not end there.
We want to take every step possible to ensure that we don’t have to stop production, recall products, or face lawsuits from harmful products, which means we don’t want to ONLY focus on purifying the water… We want to monitor how pure it is after the process.
Continuous monitoring of the water allows us to ensure that the purification system works. This involves liquid particle counting: a process that examines liquids to check for sub-visible particles.
If you want to ensure your contamination control strategy is sufficient, brush up on your particle counting knowledge in our Knowledge Center. It’s free to everyone and breaks down everything you need to know about particle counting and cleanrooms!


